Duchenne Muscular Dystrophy
-
Dystrophinopathies are a spectrum of muscle disorders which have in common deficiency of protein dystrophin.
-
The DMD gene is the longest known human gene (~2.4 Mb, 79 exons) that encodes for the 427 kDa protein dystrophin.
-
Asymptomatic increase in serum creatine phosphokinase (CK) and muscle cramps with myoglobinuria represent the milder end of dystrophinopathy spectrum, whereas Duchenne muscular dystrophy (DMD) and Becker muscular dystrophy (BMD) constitute the severe and intermediate ends, respectively.
-
DMD in boys typically manifests between 3 and 5 years of age and most kids are wheelchair-bound before 12 years.
-
The commonest DMD gene mutations are deletions (60–70%) followed by duplications (5–15%). The two regions in the DMD gene that are prone to deletions and duplications are exons 45–55 and exons 2–19. Screening of the aforementioned exons (hot spots) for the presence of mutation by multiplex ligation-dependent probe amplification (MLPA) is usually the first step in a suspected dystrophinopathy.
-
The limitation of hot spot screening is that small mutation can be missed.
-
Exon skipping is an escape phenomenon that is deployed by the myocytes to restore the altered reading frame by means of alternative splicing. This phenomenon produces a truncated but partly functional protein to restore the functional loss to a certain degree. These are the cases where molecular genetic studies and phenotypes do not match.
-
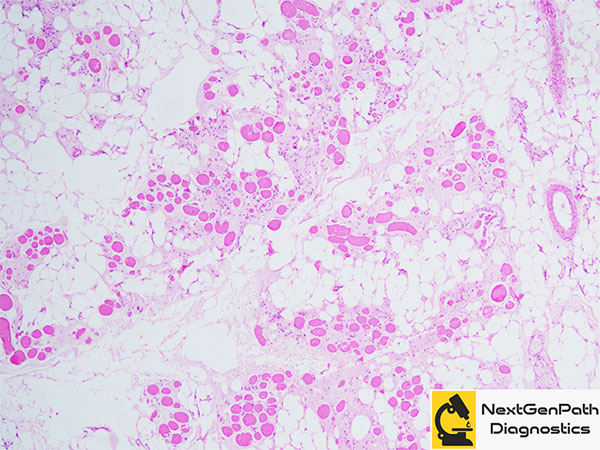
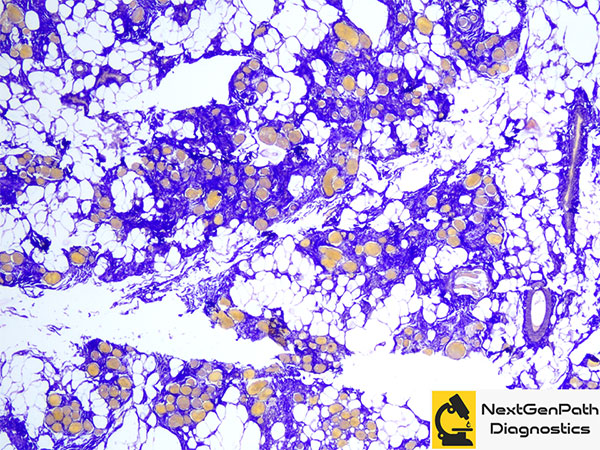
Muscle biopsy provides information with regards to the production of dystrophin and helps predict the disease phenotype as dystrophin levels on the muscle biopsy correlate better with the phenotype. Moreover, revertant fibers can only be visualized in the biopsy. For this reason, most centers do not rely exclusively on molecular genetics for the confirmation of diagnosis.
-
The first FDA approved therapy for DMD, EXONDYS 51 (ETEPLIRSEN) is an antisense oligonucleotide designed to bind to exon 51 of dystrophin pre-mRNA. This results in exclusion of this exon during mRNA processing in patients with genetic mutations that are amenable to exon 51 skipping. Exon skipping is intended to allow for production of an internally truncated dystrophin protein.